Life span, the period of time between
the birth and death of an organism.
It is a commonplace that all
organisms die. Some die after only a brief existence, like that of the mayfly,
whose adult life burns out in a day, and others like that of the gnarled
bristlecone pines, which have lived thousands of years. The limits of the life
span of each species appear to be determined ultimately by heredity. Locked
within the code of the genetic material are instructions that specify the age
beyond which a species cannot live given even the most favourable conditions.
And many environmental factors act to diminish that upper age limit.

Measurement of Life Span
The maximum life span is a
theoretical number whose exact value cannot be determined from existing
knowledge about an organism; it is often given as a rough estimate based on the
longest-lived organism of its species known to date. A more meaningful measure
is the average life span; this is a statistical concept that is derived by the
analysis of mortality data for populations of each species. A related term is
the expectation of life, a hypothetical number computed for humans from
mortality tables drawn up by insurance companies. Life expectancy represents
the average number of years that a group of persons, all born at the same time,
might be expected to live, and it is based on the changing death rate over many
past years.
The concept of life span implies that
there is an individual whose existence has a definite beginning and end. What
constitutes the individual in most cases presents no problem: among organisms
that reproduce sexually the individual is a certain amount of living substance
capable of maintaining itself alive and endowed with hereditary features that
are in some measure unique. In some organisms, however, extensive and
apparently indefinite growth takes place and reproduction may occur by division
of a single parent organism, as in many protists, including bacteria, algae,
and protozoans. If these divisions are incomplete, a colony results; if the
parts separate, genetically identical organisms are formed. In order to
consider life span in such organisms, the individual must be defined
arbitrarily since the organisms are continually dividing. In a strict sense,
the life spans in such instances are not comparable to those forms that are
sexually produced.
The beginning of an organism can be
defined by the formation of the fertilized egg in sexual forms; or by the
physical separation of the new organism in asexual forms (many invertebrate
animals and many plants). In animals generally, birth is considered to be the
beginning of the life span. The timing of birth, however, is so different in
various animals that it is only a poor criterion. In many marine invertebrates
the hatchling larva consists of relatively few cells, not nearly so far along
toward adulthood as a new-born mammal. For even among mammals, variations are
considerable. A kangaroo at birth is about an inch long and must develop
further in the pouch, hardly comparable to a new-born deer, who within minutes
is walking about. If life spans of different kinds of organisms are to be
compared, it is essential that these variations be accounted for. The end of an
organism’s existence results when irreversible changes have occurred to such an
extent that the individual no longer actively retains its organization. There
is thus a brief period during which it is impossible to say whether the
organism is still alive, but this time is so short relative to the total length
of life that it creates no great problem in determining life span.
Some organisms seem to be potentially
immortal. Unless an accident puts an end to life, they appear to be fully
capable of surviving indefinitely. This faculty has been attributed to certain
fishes and reptiles, which appear to be capable of unlimited growth. Without
examining the various causes of death in detail (see death) a distinction can
be made between death as a result of internal changes (i.e., aging) and death
as a result of some purely external factor, such as an accident. It is notable
that the absence of aging processes is correlated with the absence of
individuality. In other words, organisms in which the individual is difficult
to define, as in colonial forms, appear not to age.
Plants
Plants grow old as surely as do
animals. However, a generally accepted definition of age in plants has not yet
been realized. If the age of an individual plant is that time interval between
the reproductive process that gave rise to the individual and the death of the
individual, the age attained may be given readily for some kinds of plants but
not for others.
Problem of defining age
An English oak that has 1,000 annual
rings in the trunk is 1,000 years old. But age is less certain in the case of
an arctic lupine that germinated from a seed that, containing the embryo, had
been lying in a lemming’s burrow in the arctic permafrost for 10,000 years.
The mushroom caps that appear
overnight last for only a few days, but the network of fungus filaments in the
soil (the mycelia) may be as old as 400 years. Because of important differences
in structure, the life span of higher plants cannot be compared with that of
higher animals. Normally, embryonic cells (that is, cells capable of changing
in form or becoming specialized) cease to exist very early in the life of an
animal. In plants, however, embryonic tissue—the plant meristems—may contribute
to growth and tissue formation for a much longer time, in some cases throughout
the life of the plant. Thus the oldest known trees, bristlecone pines of
California and Nevada, have one meristem (the cambium) that has been adding
cells to the diameter of these trees for, in many cases, more than 4,000 years
and another meristem (the apical) that has been adding cells to the length of
these trees for the same period. These meristematic tissues are as old as the
plant itself; they were formed in the embryo. The wood, bark, leaves and cones,
however, live for only a few years. The wood of the trunk and roots, although
dead, remains a part of the tree indefinitely, but the bark, leaves, and cones
are continually in the process of dying and sloughing off.
Among the lower plants only a few
mosses possess structures that enable an estimate of their age to be made. The
haircap moss (Polytrichum) grows through its own stem tip each year, leaving a
ring of scales that marks the annual growth. Three to five years’ growth in
this moss is common, but life spans of 10 years have been recorded. The lower
portions of such a moss are dead, though intact. Peat moss (Sphagnum) forms
extensive growths that fill acid bogs with a peaty turf consisting of the dead
lower portions of mosses whose living tops continue growing. Mosses that become
encrusted with lime (calcium carbonate) and form “tufa” beds several metres
thick also have living tips and dead lower portions. On the basis of their
observed annual growth, some tufa mosses are estimated to have been growing for
as long as 2,800 years.
No reliable method for determining
the age of ferns exists, but on the basis of size attained and growth rate,
some tree ferns are thought to be several decades old. Some club mosses, or
lycopsids, have a “storied” growth pattern similar to that of the haircap moss.
Under favourable conditions some specimens live five to seven years.
The woody seed plants, such as
conifers and broadleaf trees, are the most amenable to determination of age. In
temperate regions, where each year’s growth is brought to an end by cold or
dryness, every growth period is limited by an annual ring—a new layer of wood
added to the diameter of the tree. These rings may be counted on the cut ends
of a tree that has been felled or, using a special instrument, a cylinder of
wood can be cut out and the growth rings counted and studied. In the far north
growth rings are so close together that they are difficult to count. In the
moist tropics growth is more or less continuous, so that clearly defined rings
are difficult to find.
Often the age of a tree is estimated
on the basis of its diameter, especially when the average annual increase in
diameter is known. The source of greatest error in this method is the not
infrequent fusing of the trunks of more than one tree, as, for example,
occurred in a Montezuma cypress in Santa María del Tule, a little Mexican
village near Oaxaca. This tree, described by the Spanish explorer Hernan Cortés
in the early 1500s, was earlier estimated on the basis of its great thickness
to be 6,000 years old; later studies, however, proved it to be three trees
grown together. Estimates of the age of some English yews have been as high as
3,000 years, but these figures, too, have turned out to be based on the fusion
of close-growing trunks, none of which is more than 250 years old. Increment
borings of bristlecone pines have shown specimens in the western United States
to be 4,600 years old.
Growing season of seed plants
Annuals
Plants, usually herbaceous, that live
for only one growing season and produce flowers and seeds in that time are
called annuals. They may be represented by such plants as corn and marigolds,
which spend a period of a few weeks to a few months rapidly accumulating food
materials. As a result of hormonal changes—brought about in many plants by
changes in environmental factors such as day length and temperature—leaf-producing
tissues change abruptly to flower-producing ones. The formation of flowers,
fruits, and seeds rapidly depletes food reserves and the vegetative portion of
the plant usually dies. Although the exhaustion of food reserves often accompanies
death of the plant, it is not necessarily the cause of death.
Biennials
These plants, too, are usually
herbaceous. They live for two growing seasons. During the first season, food is
accumulated, usually in a thickened root (beets, carrots); flowering occurs in
the second season. As in annuals, flowering exhausts the food reserves, and the
plants die after the seeds mature.
Perennials
These plants have a life span of
several to many years. Some are herbaceous (iris, delphinium), others are
shrubs or trees. The perennials differ from the above-mentioned groups in that
the storage structures are either permanent or are renewed each year.
Perennials require from one to many years growth before flowering. The
preflowering (juvenile) period is usually shorter in trees and shrubs with
shorter life spans than in those with longer life spans. The long-lived beech
tree (Fagus sylvatica), for example, passes 30–40 years in the juvenile stage,
during which time there is rapid growth but no flowering.
Some plants—cotton and tomatoes, for
example—are perennials in their native tropical regions but are capable of
blooming and producing fruits, seeds, or other useful parts in their first
year. Such plants are often grown as annuals in the temperate zones.
Longevity of seeds
Although there is great variety in
the longevity of seeds, the dormant embryo plant contained within the seed will
lose its viability (ability to grow) if germination fails to occur within a
certain time. Reports of the sprouting of wheat taken from Egyptian tombs are
unfounded, but some seeds do retain their viability a long time. Indian lotus
seeds (actually fruits) have the longest known retention of viability. On the
other hand, seeds of some willows lose their ability to germinate within a week
after they have reached maturity.
The loss of viability of seeds in
storage, although hastened or retarded by environmental factors, is the result
of changes that take place within the seed itself. The changes that have been
investigated are: exhaustion of food supply; gradual denaturing or loss of
vital structure by protoplasmic proteins; breakdown of enzymes; accumulation of
toxins resulting from the metabolism of the seed. Some self-produced toxins may
cause mutations that hamper seed germination. Since seeds of different species
vary greatly in structure, physiology, and life history, no single set of age
factors can apply to all seeds.
Animals
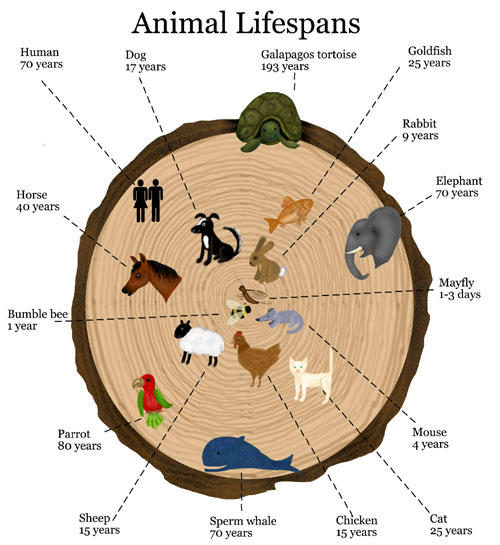
Much of what is known of the length
of life of animals other than man derives from observations of domesticated
species in laboratories and zoos. One has only to consider how few animals
reveal their age to appreciate the difficulties involved in answering the
apparently simple question of how long they live in nature. In many fishes, a
few kinds of clams, and an occasional species of other groups, growth is
seasonal, so that annual zones of growth, much like tree rings, are produced in
some part of the organism. Among game species, methods of determining relative
age by indicators such as the amount of tooth wear or changes in bone structure
have yielded valuable information. Bird bands and other identifying marks also
make age estimation possible. But one of the consequences of the fact that
animals move is that very little is known about the life span of most species
as they exist in nature.
Maximum and average longevity
Many of the extreme claims of
longevity that are occasionally made for one species or another have
consistently been proven false when subjected to critical scrutiny. Although
the maximum life span that has been observed for a particular species cannot be
considered absolute, since a limited number of individuals at best has been
studied, this datum probably provides a fair approximation of the greatest age
attainable for this kind of animal under favourable conditions. Animals in
captivity, which provide most of the records of extreme age, are exposed to far
fewer hazards than those in the wild.
Environmental influences
Life span usually is measured in
units of time. Although this may seem eminently logical, certain difficulties
may arise. In cold-blooded animals in general, the rate of metabolism that
determines the various life processes varies with the temperatures to which
they are exposed. If aging depends on the expenditure of a fixed amount of
vital energy, an idea first proposed in 1908, life span will vary tremendously
depending on temperature or other external variables that influence life span.
There is considerable evidence attesting at least to the partial cogency of
this argument. So long as a certain range is not exceeded, cold-blooded
invertebrates do live longer at low than at high temperatures. Rats in the
laboratory live longest on a somewhat restricted diet that does not permit
maximum metabolic rate. Of perhaps even greater significance is the fact that
many animals undergo dormant periods. Many small mammals hibernate; a number of
arthropods have life cycles that include periods during which development is
arrested. Under both conditions the metabolic rate becomes very low. It is
questionable whether such periods should be included in computing the life span
of a particular organism. Comparisons between species, some of which have such
inactive periods while others do not, are dangerous. It is possible that life
span could be measured more adequately by total metabolism; however, the data
that are necessary for this purpose are almost entirely lacking.
Length of life is controlled by a
multitude of factors, which collectively may be termed environment, operating
on a genetic system that determines how the individual will respond. It is
impossible to list all the environmental factors that may lead to death. For
analytical purposes it is, however, useful to make certain formal separations.
Every animal is exposed to (1) a pattern of numerous events, each with a
certain probability of killing the individual at any moment and, in the
aggregate, causing a total probability of death or survival; (2) climatic and
other changes in the habitat, modifying the frequency with which the various
potentially fatal events occur; and (3) progressive systemic change, inasmuch
as growth, reproduction, development, and senescence are characteristics
intrinsic in the organism and capable of modifying the effects of various environmental
factors.
Patterns of survival
Consider a group of similar animals
of the same age. Although no two individuals can have precisely the same
environment, let it be assumed that the environment of the group remains
effectively constant. If the animals undergo no progressive physiological
changes, the factors causing death will produce a death rate that will remain
constant in time. Under these conditions, it will take the same amount of time
for the population to become reduced to one-half its former number, no matter
how many animals remain at the beginning of the period considered. The animals
therefore survive according to the pattern of an accident curve. This is the
sense in which many of the lower animals are immortal. Although they die, they
do not age; how long they have already lived has no influence on their further
life expectation.
Another group of animals may consist
of individuals that differ markedly in their responses to the constant
environment. They may be genetically different, or their previous development
may have caused variations to arise. Those individuals that are most poorly
suited to the new environment will die, leaving survivors that are better
adapted. The same result can also be achieved in other ways. If the environment
varies geographically, those individuals that happen to find areas in which
existence can be maintained will survive, while the remainder will die. Or, as
a result of their own properties, animals in a constant environment may
acclimate in a variety of ways, thus adjusting to the existing conditions. The
pattern of survival that results in each of these cases is one in which the
death rate declines with time, as illustrated by the selection–acclimation
curve.
In the absence of death from other
causes, all members of a population may exist in their environment until the
onset of senescence, which will cause a decline in the ability of individuals
to survive. In a sense they can be considered to wear out as does a machine.
Their survival is best described by individual differences among members of the
population that determine the curvature of the survival line (wearing-out
curve). The more the population varies, the less abrupt is the transition from
total survival to total death.
Under the actual conditions of
existence of animals the three types of survival (accident pattern,
selection–acclimation pattern, wearing-out pattern) above all enter as
components of the realized survival pattern. Thus in animals that are carefully
maintained in the laboratory, survival is approximately that of the wearing-out
pattern. Environmental accidents can be kept to a minimum under these
conditions, and survival is almost complete during the major part of the life
span. In all known cases, however, the early stages of the life span are
characterized by a noticeable contribution of the selection–acclimation
pattern. This must be interpreted as a result of developmental changes that
accompany the early life of the individuals and of selective processes that
operate on those organisms whose genetic constitutions are ill fitted for that
environment.
In some of the larger mammals in
nature, the existing evidence points to a similar survival pattern. In a
variety of other animals, however, and including fishes and invertebrates,
mortality in the young stages is so high that the selection–acclimation curve
predominates. One estimate places the mortality of the Atlantic mackerel during
its first 90 days of life as high as 99.9996 percent. Since some mackerel do
live for several years, a mortality rate that decreases with age is indicated.
Similar considerations probably apply to all those animals that have larval
stages that serve as dispersal mechanisms.
When the postjuvenile portion of the
life span is considered by itself, a number of animals for which such
information has been gathered—including primarily fishes and birds—have
survivorship curves that are dominated by the accident pattern. In these species
in nature, death from old age apparently is rare. Their chance of surviving to
an advanced age is so small that it may be statistically negligible. In modern
times, human predation is a large factor in the mortality of these species in
many cases. Since deaths from fishing and hunting are largely independent of
age, once an animal has reached a certain minimum size, such a factor only
makes the survival curve steeper but does not change its shape. One consequence
of such increased mortality is that fewer old and large individuals are noticed
in a population.
More complex survival patterns, such
as the hypothetical one illustrated, undoubtedly exist. They should be looked
for in those species in which extensive reorganization of the animal is part of
the normal life cycle. In effect, these animals change their environment
radically, in some cases several times during a lifetime. The frog offers a
familiar example. During its period of early development and until shortly
after hatching, the animal is subject to major internal, and some external,
change. As a tadpole it is adjusted to an aquatic, herbivorous life. The
metamorphosis to the terrestrial, carnivorous adult form is accompanied by
varied physiological stresses that must be expected to produce a temporary
increase in mortality rate. In some insects the eggs, larvae, pupae, and adults
are exposed to and respond to quite different environments, and a survivorship
pattern even more complex than that described by the composite curve may exist.
The same species will exhibit changed
survival in different environments. In captivity an animal population may
approach the wearing-out pattern; in its natural habitat survivorship may vary
with age in a quite different way. Although one can assign a maximum potential
life span to an individual—while realizing that this maximum may not be
attained—it is impossible to specify the survivorship pattern unless the
environment is also specified. This is another way of saying that life span is
the joint property of the animal and the environment in which it lives.
Comments
Post a Comment